European Foulbrood Test Kit
The Vita European foulbrood (EFB) Diagnostic Test Kit enables beekeepers and bee inspectors to test for EFB in honeybee larvae and to obtain the results immediately and easily in the apiary.
Key Facts
- Works like a home pregnancy test kit and reacts specifically to antibodies associated with the pathogen Melissococcus plutonius which causes EFB in honeybees.
- The kit performs as well as standard EFB laboratory tests. It has been validated by the National Bee Unit of the Food and Environment Research Agency (FERA), York and at other institutes internationally. It has been developed for Vita by the Foresite Diagnostics section at FERA in York, UK.
- The EFB test kit is similar to and complements the AFB Diagnostic Test Kit (which tests for Paenibacillus larvae subsp. larvae, which causes AFB in honeybees).
Why use Vita's EFB diagnostic kit
Vita's EFB field diagnostic kit is specific to Melissococcus plutonius and takes just three minutes to give a result, validated to 98%+ accuracy.
The kit is extremely simple to use in the field and requires no samples to be sent away to laboratories. Anyone can use Vita's EFB diagnostic kit to confirm or allay their suspicions of infection.
Vita's EFB diagnostic kit has been rigorously tested for use in the field. It has been validated at the National Bee Unit of the Food and Environment Research Agency (FERA) in the UK and at other institutes internationally. The Kit enables beekeepers to test their hives at the first suspicion of EFB and is an ideal tool in the training of beekeeping experts.
When to use Vita's EFB Diagnostic Kit
When testing for European Foulbrood, it is best to extract young suspect larvae, as in older larvae other agents may mask the antibodies released by Melissococcus plutonius. The kit should be used at the first suspicion of an EFB infection.
How to use Vita's EFB Diagnostic Kit
- Whole or part samples can be used but it is recommended that a whole infected larva be used for best results.
- Extract larva showing suspicious symptoms with Spatula.
- Unscrew lid from Extraction Bottle. Use Spatula to deposit Sample in Bottle. Replace lid and shake vigorously for about 20 seconds.
- Remove Test Device from foil pack. WARNING: do not touch Viewing Window.
- Unscrew lid of Extraction Bottle and use supplied Pipette to remove Sample from Bottle. For best results remove Sample immediately after shaking to prevent bacteria from settling out of suspension.
- Hold Test Device horizontally and gently squeeze two drops onto the sample well of Device.
- Keep device horizontal until extract is absorbed (c. 30 seconds) and a blue dye appears in Viewing Window.
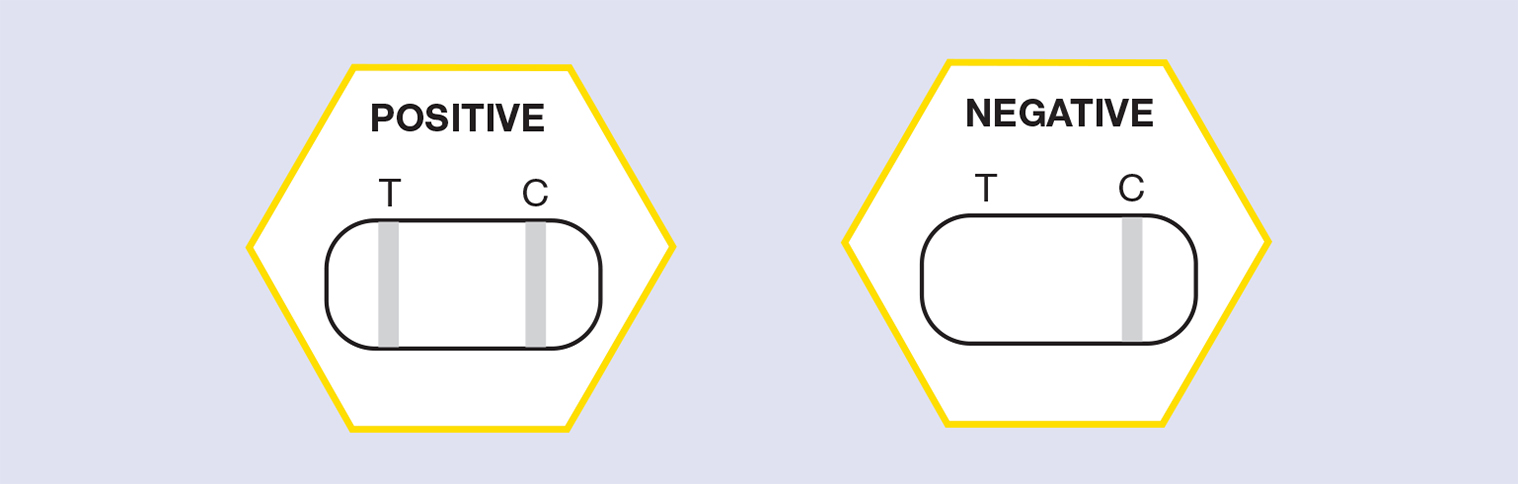
- Wait until Control Line Appears (labelled C) and read the result (c. 1-3 minutes) Interpreting the Results.
Kit Contents
Reading the results
- A positive result (two lines show up – both Test and Control, see below) indicates that the target pathogen is present in the test sample, that is that the cell sampled contained infection by European Foulbrood.
- A negative result (Control line shows up only, no Test line) indicates that the EFB pathogen has not been detected in the test sample.
- There is space on the label on the reverse of the device to write details such as sample identification, date and result. Also on the reverse is a batch number for each device. If problems are encountered with any device please quote this batch number when contacting Vita (Europe) Limited.
- Please dispose of your test carefully and help to keep the countryside tidy.
- Which is correct – above, below or both?
- Two blue lines (C&T) indicates a positive result, test performed OK.
- One blue line (C only) indicates a negative result, test performed OK.
- Faint blue T line, strong C line indicates a possible positive, test performed O.K.
- One blue line (T only) indicates the test has failed
- No lines present indicates the test has failed
- Brown C or T lines indicates the test has failed.
If the test has failed we recommend carrying out another test using a new sample from the same original comb with a new device. Should problems persist please contact us.
Note: As with all diagnostic testing a negative reaction does not necessarily indicate that the target pathogen is absent. A faint or absent line may indicate a low concentration of the pathogen or recent infection. If in doubt, repeat with a new device using a fresh sample or repeat in a few days time.
Store at room temperature, use as soon as possible after opening foil pack and keep Test Device dry at all times.